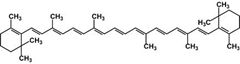
Beta-carotene (b-carotene; BAY-tuh KARE-oh-teen) belongs to a family of organic compounds called the carotenoids. The carotenoids are all brightly pigmented (colored) compounds found in a number of plants, bacteria, algae, and fungi. Betacarotene is responsible for the yellowish to orange color of pumpkins, apricots, sweet potatoes, nectarines, and, most notably, carrots. The compound also occurs in spinach and broccoli, but in such small concentrations that the green chlorophyl presentmasks the orange color of beta-carotene. In its pure form, beta-carotene occurs as purple crystals shaped like thin leaflets.
In plants, algae, and photosynthetic bacteria, beta-carotene plays an important role in photosynthesis, the process by which plants convert water and carbon dioxide into carbohydrates and oxygen. In nonphotosynthetic bacteria and fungi, beta-carotene protects the organism against the harmful effects of light and oxygen.
Animals require beta-carotene for normal growth and development, but are unable to manufacture the compound themselves. As a result, they must ingest some beta-carotene from plant sources in order to stay healthy. The compound is a provitamin, a substance that is converted in the body to a vitamin. Beta-carotene is converted into vitamin A, whose role in the body is the maintenance of strong bones and teeth and healthy skin and hair. Beta-carotene also acts as an antioxidant, a substance that attacks free radicals in the body that may cause cancer. It may also protect against heart disease and strengthen the body’s immune system.
Beta-carotene was first isolated by the German chemist Heinrich Wilhelm Ferdinand Wackenroder (1789–1854), who extracted the compound from carrot roots in 1831. The compound was first synthesized in 1950 by the Swiss chemist Paul Karrer (1889–1971).
Beta-carotene can be obtained from natural sources by crushing or pulverizing the source (such as carrots) and adding a solvent that will dissolve the organic components of the plant. These components can then be separated from each other by chromatographic techniques. A major commercial source of beta-carotene obtained by this method is the algae Dunaliella salina, which grows in large salt lakes in Australia. The compound can also be prepared synthetically by one of two methods, the BASF and the Roche methods, both named after the pharmaceutical firms where they were developed. Both methods of preparation begin with long-chain hydrocarbons containing about twenty carbon atoms each. These hydrocarbons are then joined to each other to form the 40-carbon beta-carotene compound.
Beta-carotene has two uses: in vitamin supplements and as a food additive. Anyone who eats a healthy diet that includes foods rich in vitamin A, such as fish oil, liver, eggs, butter, and orange or yellow vegetables and fruits, will get adequate amounts of beta-carotene. However, many people take vitamin supplements to ensure that they have enough beta-carotene (as well as other vitamins) in their daily diet. Although some warnings have been issued about taking too much vitamin A, there is no clinical evidence that an overdose of the vitamin does any long-term harm to a person.
Beta-carotene is used as a food additive to increase the color intensity of a product. It is used primarily with yellow and orange foods, such as butter and margarine, although it is sometimes added to ice cream and fruit juices as well. Beta-carotene is used in only very small amounts as a food additive. In these amounts, it poses no health hazard to humans or other animals. The compound has also been used in experiments to test its effectiveness against certain diseases, such as lung cancer. In such cases, it has been found to be more harmful than beneficial, increasing the risk of cancer and death among people participating in the studies.